Abstract
Introduction: Following allogeneic hematopoietic stem cell transplant (allo-HCT), donor T cells react against host antigens and cause severe graft versus host disease (GvHD) (Majhail N.S. et al., 2015. Biol Blood Marrow Transplant). Disruption of gut integrity is one of the major contributors to GvHD morbidity and mortality. Dysbiosis due to GvHD decreases host immunity and increases transplant-related mortality (Taur Y. et al., 2014. Blood). Indole, a bacterial metabolite, and indole derivatives are known to ameliorate host immunity and gut epithelial barrier function through modulating goblet cells, innate lymphoid cells (ILCs), dendritic cells (DCs) and T cell subsets (Gutiérrez-Vázquez C. & Quintana F.J., 2018. Immunity; Hubbard T.D. et al., 2015.Sci Rep). Indoles act via the aryl hydrocarbon receptor (AHR) expressed in innate lymphoid cells to generate regulatory T cells (Tregs) while inhibiting Th1 polarization (Gutiérrez-Vázquez C. & Quintana F.J., 2018. Immunity; Hubbard T.D. et al., 2015. Sci Rep). Previously, we showed that enteral administration of indole 3-carboxaldehyde (ICA) to murine allo-transplant recipients reduces GvHD severity and mortality (Swimm A. et al., 2018. Blood). However, since ICA is not available as a GMP-compliant FDA- approved pharmaceutical, our goal is to identify indoles for clinical research that have protective effects against GvHD. Here, we report that indole-3-carbinol (I3C), an indole currently marketed as a nutritional supplement, induces Treg generation and higher survival in allo-HCT.
Methods: To study modulation of T cell subsets by indoles, mice were gavaged daily for 14 days with corn-oil suspensions of 50, 100, 200 or 400mg/kg of I3C, 150mg/kg of ICA, or corn oil alone. On day 15, mice were euthanized and splenocytes were isolated to study T cell subsets. To investigate the effect of I3C on GvHD, lethally irradiated (9Gy) B10.BR (H-2Kk) mice received 5 x 106 T cell-depleted bone marrow (TCDBM) cells with or without 2 x 106 T cells from MHC mismatched C57BL/6 (H-2Kb) donors. Allo-transplanted mice received either vehicle or 200mg/kg of I3C daily from day -2 to day 40. Mice were weighed and assessed twice a week for first 30 days and once a week afterwards. Lethally irradiated (9Gy) C57BL/6 mice were received 5 x 106 TCDBM cells with or without 5 x 105 T cells from FVB (H2Kq) donors and 14 days of daily gavage with vehicle or 200 mg/kg of I3C or 150mg/kg of ICA to study gut bacterial translocation into mesenteric lymph nodes (MLN). On the following day, MLNs were collected, immediately homogenized in 0.25ml sterile PBS and cultured (100ul) on Blood Agar plate to determine CFU/g tissue.
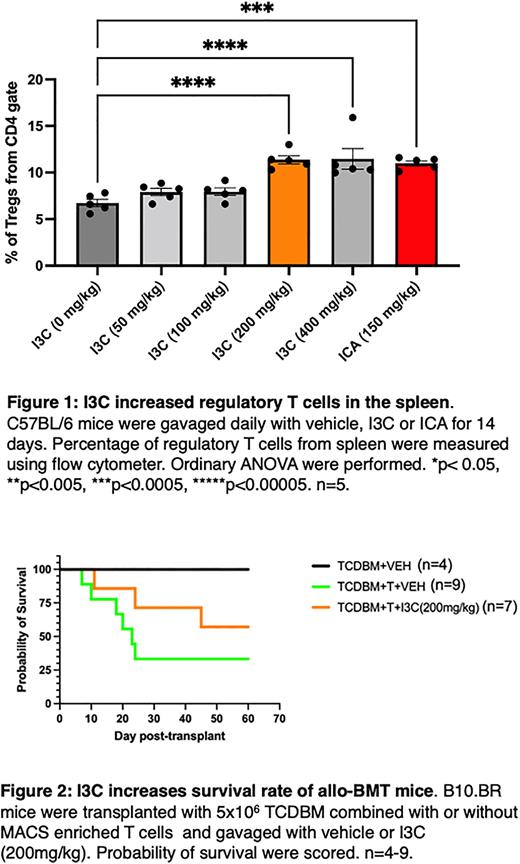
Results: Percentages of regulatory T cells (Tregs) in the spleen were significantly increased at 14 days in non-transplanted mice treated with 200mg/kg of I3C (11.38%, n=5), 400mg/kg of I3C (11.48%%, n=5) and ICA (11%%, n=5) compared to mice treated with vehicle (6.74%%, n=5) (Figure 1), whereas frequencies of CD8+ T cells were unchanged. Allo-transplanted mice treated with I3C had higher survival (57.14%, n=7) compared to the vehicle-treated control group (33.33%, n=9) (Figure 2). However, weight gain was slower in the I3C-treated mice compared to surviving vehicle-treated mice. Allo-transplanted recipient mice showed trend of reduced bacterial translocation into MLNs of mice that received 200mg/kg of I3C and ICA. Our results indicate I3C supplement enhances rate of survival and decreases gut bacterial translocation into MLNs in allo-HCT recipients. Continuing experiments will study bacterial composition, histological changes, and determine mechanisms for I3C restoration of gut integrity in all-HCT recipients.
Conclusion: The results above suggest indoles increase regulatory T cells. Allo-bone marrow transplant recipients treated with indoles had improved survival. We propose I3C as a prospective supplement to treat GvHD in all-HCT patients.
Keywords: Allo-HCT, GvHD, Indoles, I3C, Tregs, Survival
Acknowledgement: Research reported in this publication was supported in part by the Morningside Center for Innovative and Affordable Medicine through the generous support of donors and Emory University.
Disclosures
Waller:Verastem Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orca Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.